![]()
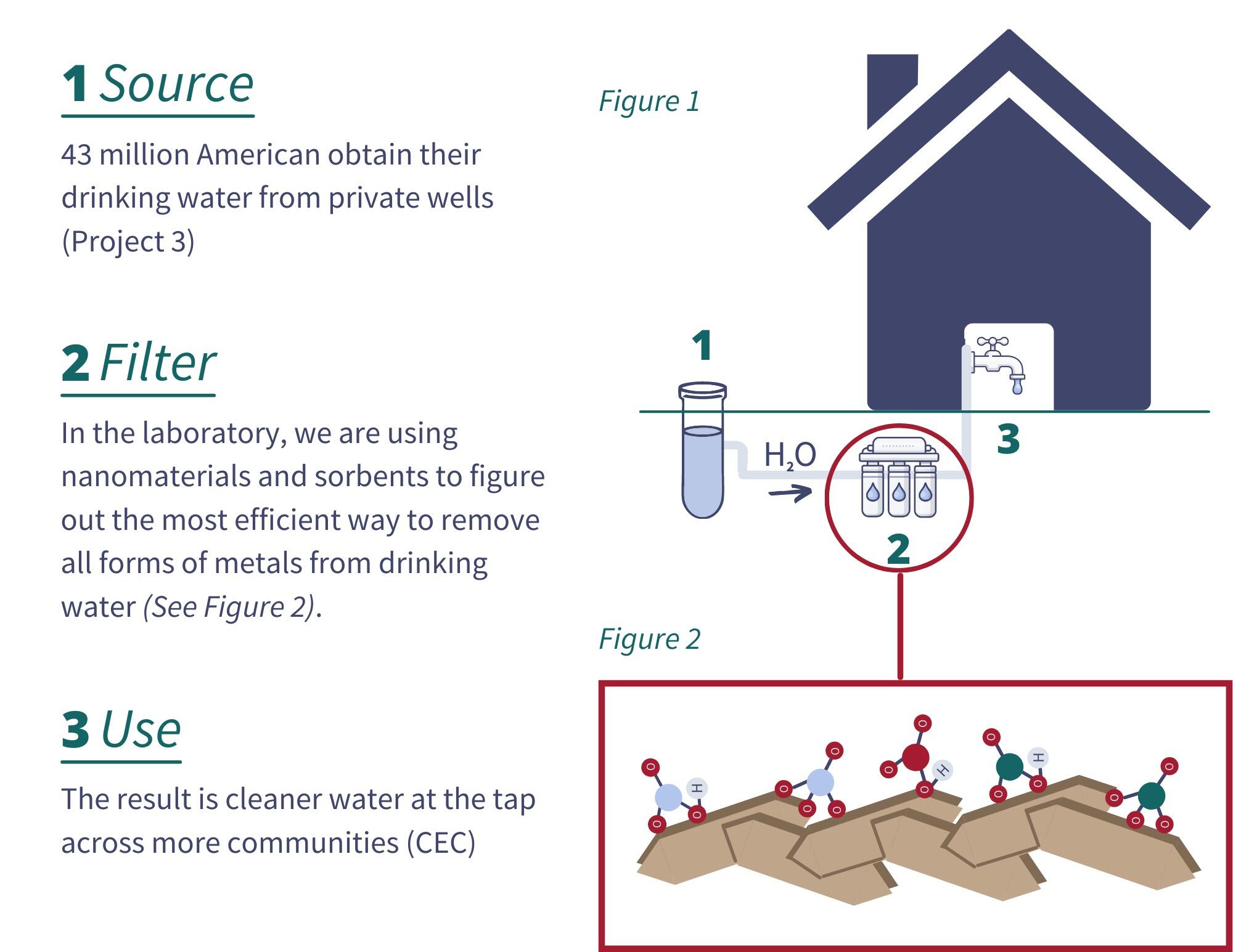 Removing metals and other chemicals that can contaminate drinking water is critical for public health, but traditional approaches to filter and remove neurotoxic metals are not sufficiently selective in the presence of other non-toxic ions, which frequently occur at higher concentrations, have similar chemical structures, and compete for sorption sites.
Removing metals and other chemicals that can contaminate drinking water is critical for public health, but traditional approaches to filter and remove neurotoxic metals are not sufficiently selective in the presence of other non-toxic ions, which frequently occur at higher concentrations, have similar chemical structures, and compete for sorption sites.
Our Goals
This project aims to develop a drinking water filter technology that
- selectively removes neurotoxic oxo-anions from drinking water by adsorption
- can be used for a variety of water treatment systems (e.g., individual households, on well water, small-scale community systems or schools)
- are more effective, efficient, and sustainable than existing technologies.
Our Approach
Recent advances in polymer- and nano- science allow for unprecedented bottom-up capabilities to thermodynamically model, characterize, and controllably synthesize adsorbents. Project 4 is designing selectively adsorptive polymers based on the waste bio-polymer chitosan and metal oxides particles. By selectively exposing different surfaces of the metal oxide particles, we are able to exploit chemical behavioral differences such as polarity, charge distribution, size, and hydrophobicity between the target oxoanion metal pollutants and other naturally occurring competing ions, to generate highly selective and tunable polymeric and nano-surfaces.







